Clinical Background
Bacterial vaginosis (BV) is the most common of all vaginal conditions that bring women to healthcare providers. It is characterized by a loss of beneficial normal flora (Lactobacilli) and an overgrowth of Gram-negative anaerobes and Actinobacteria. Women with BV are at greater risk of intrauterine and placental infections, preterm labor with delivery of premature low birth weight infants, endometritis, and pelvic inflammatory disease. They are also at greater risk of acquiring sexually transmitted infections and urinary tract infections.
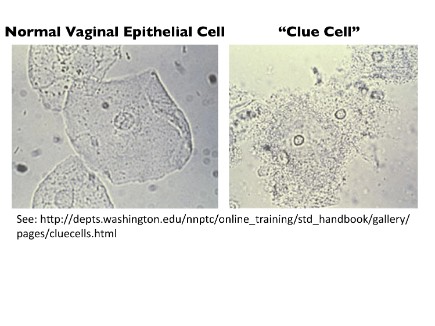
The clinical criteria for BV (Amsel critera), include 'thin' vaginal fluid, fishy odor upon potassium hydroxide treatment, elevated vaginal pH (>4.5) attributed to reduced lactic acid bacteria, and microscopic examination demonstrating exfoliated epithelial cells that are studded with attached bacteria (“clue cells” see Figure). Conventional treatments are often followed by recurrences of BV and little progress has been made improving pregnancy outcomes associated with BV.
BV Sialidases
BV is frequently characterized by the presence of sialidase activity in vaginal fluids. In fact, sialidase activity is now used as a diagnostic feature of BV.
In general, sialidases remove the outermost sialic acid residues from sugar chains. However, the potential roles of sialidases in BV are not fully understood.
Secretory IgA is a substrate of BV-associated glycosidases
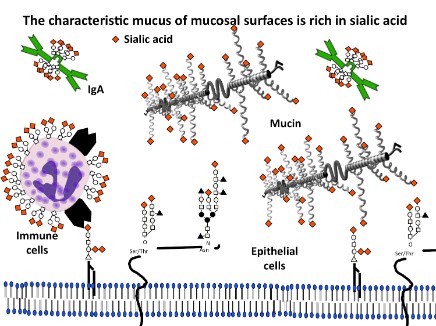
We have recently demonstrated that secretory IgA (SIgA) is a relevant substrate of BV-associated sialidases in clinical specimens (see Figure). BV-associated beta-galactosidases and N-acetyl hexosaminidases also acted on SIgA in clinical samples, exposing otherwise cryptic terminal mannose residues on secretory component (SC). Together, these experiments implicate sialic acids as a first line of biochemical defense against deglycosylation of SIgA and possibly other heavily glycosylated mucus components in the reproductive tract. Biochemical models illustrated processive exo-deglycosylation of SC and showed that gentle partial deglycosylation of the heavily glycosylated SC enhances its susceptibility toward proteolysis (see Figure), likely by removal of steric hindrance provided by the glycan. The evidence supports a model of BV in which elevated protease activity together with multiple exo-glycosidase enzymes may produce a state of hydrolysis and depletion of important mucosal sialoglycoproteins such as SIgA.
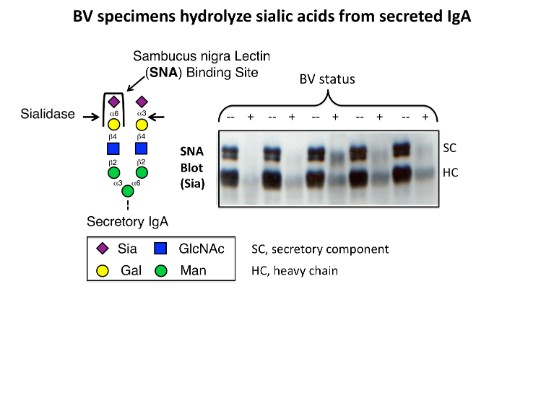
BV sialidases act on sialic acids in 2-3 and 2-6 linkages of N- and O-glycans
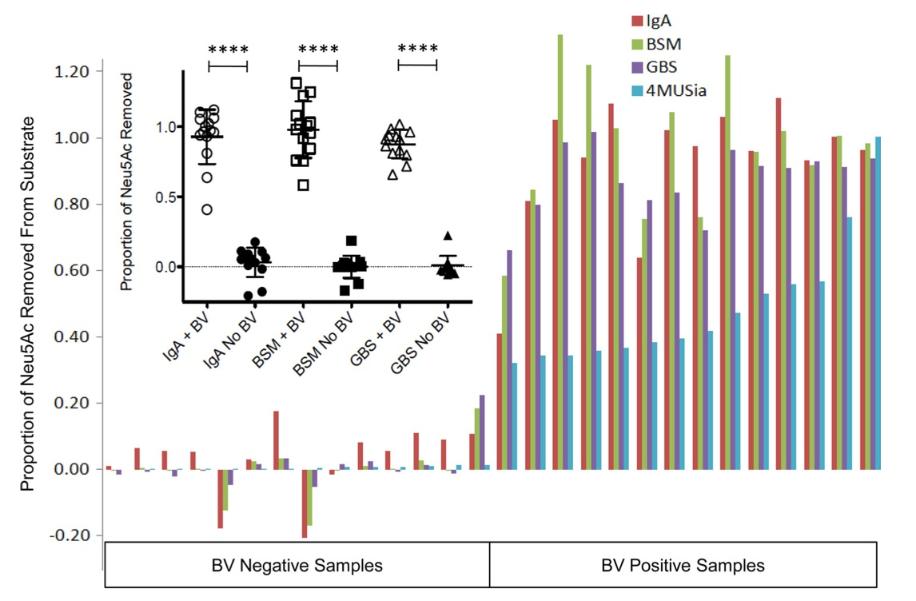
To examine the specificity of BV sialidases, vaginal specimens of women with BV were incubated together with various potential sialoglycan substrates: 1) IgA, which contains sialic acids mostly in a2-6 linkages of N-glycans, 2) bovine submaxillary mucin, which contains sialic acids in a2-6 and a2-3 linkages mostly on O-glycans, and 3) the Group B streptococcal capsular polysaccharide, which has exclusively a2-3 linked sialic acid residues. We show that sialidase activity (4MUSia assay) in vaginal fluids from women with BV (n=13 individuals) cleaves 90-100% of sialic acids from all of these substrates.
Lewis Lab Papers related to BV and sialidases
Gilbert, N.M.; Lewis, WG; Lewis, AL. Clinical Features of Bacterial Vaginosis in a Murine Model of Vaginal Infection with Gardnerella vaginalis. PLoS One. 2013;8(3):e59539. doi: 10.1371/journal.pone.0059539. Epub 2013 Mar 19.
Lewis, WG; Robinson, LS; Gilbert, N.M.; Perry, JC; Lewis, AL. Degradation, Foraging and Depletion of Mucus Sialoglycans by the Vagina-Adapted Actinobacterium Gardnerella vaginalis. J. Biol. Chem. 2013 Apr 26;288(17):12067-79. doi: 10.1074/jbc.M113.453654. Epub 2013 Mar 11.
Lewis, WG; Robinson, LS; Perry, JC; Peipert, JF; Allsworth, JE; Lewis, AL. Hydrolysis of the Secreted Sialoglycoprotein Immunoglobulin A (IgA) in Ex Vivo and In Vitro Models of Bacterial Vaginosis. J. Biol. Chem. 2012. Jan 13;287(3):2079-89. http://www.ncbi.nlm.nih.gov/pubmed/22134918