Sialic acid Mimicry
A number of human pathogens mimic sialic acids, using several distinct mechanisms to suppress or misdirect host immune responses. For example, high density expression of sialic acid residues allows several bacterial pediatric pathogens to survive and multiply in the bloodstream. Our recent work suggests that expression of sialic acids and related nonulosonic acids (NulOs) among bacteria is much more common than previously realized.
Sialic Acids, Legnionaminic Acids, Pseudaminic Acids
[2] We here use the abbreviation NulO for Non-2-ulosonic acids, which assumes Nul for Non-2-uloses and maintains the discrimination between aldonic acids and uronic acids such as Glucuronic acid (GlcA). We suggest that the use of the term “sialic acid" (Sia) continue to be limited to their original use in describing Neu, Kdn and their derivatives in deuterostomes and their pathogens, and that “NulO” be used to encompass the entire group of nine carbon alpha-keto acids.
Diverse nonulosonic acids (NulOs) are synthesized by related biochemical pathways
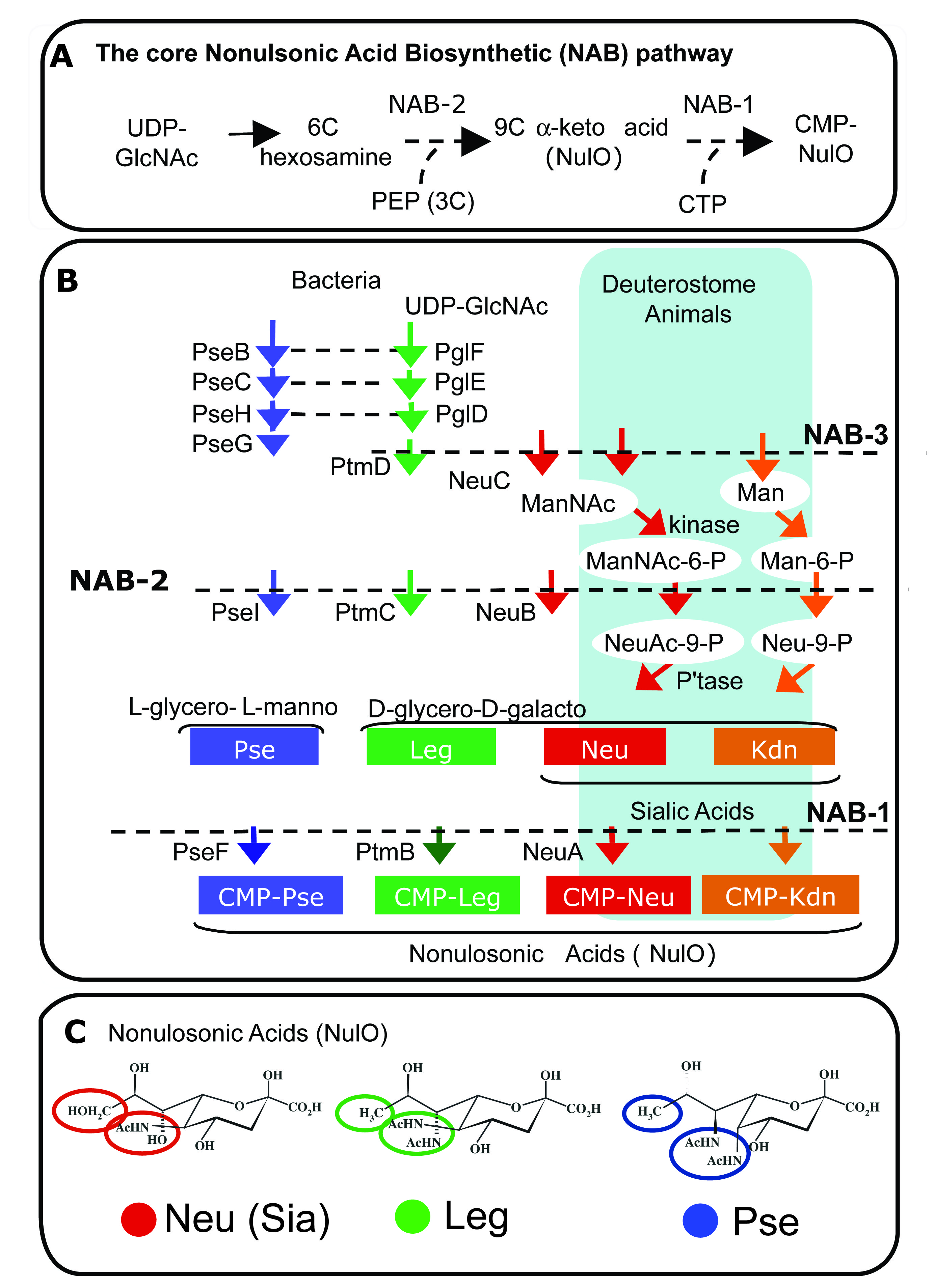
Phylogenetic Prediction of NulO structure (Lewis PNAS, 2009)
We used approaches in genomics and phylogenetics together with targeted biochemical validation to make predictions of carbohydrate structure (i.e. type of NulO) based on genomic sequences. This approach revealed that biosynthesis of NulOs was far more common than previously realized.
Phylogenetic analysis of NulO pathways revealed that sialic acids (depicted in red clades in the figure to the right) were 'invented' independently at least 3 times by bacteria. Multiple lines of evidence suggest that one of these inventions may have involved adaptation of a bacterial pathway for legionaminic acid synthesis (clade shown in green), a NulO molecule with the same stereochemistry as sialic acid.
This approach also revealed new potential examples of sialic acid mimicry among bacterial pathogens, a known virulence factor in other contexts that enables pathogens to cause both localized and systemic infections.
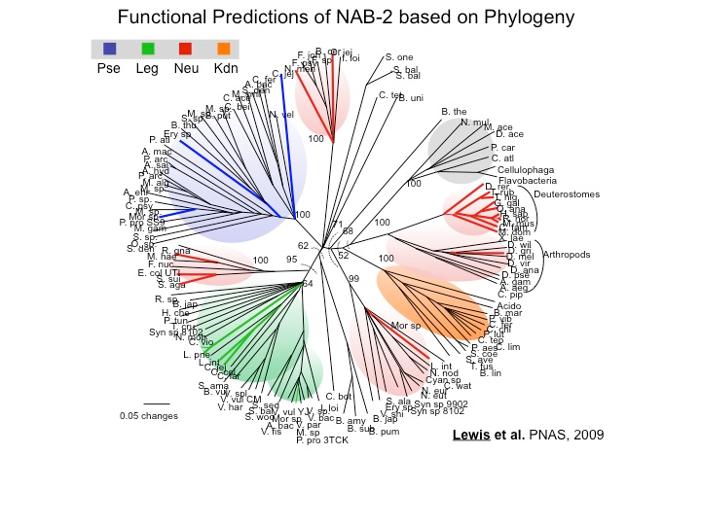
Sialic acid mimicry by Vibrio vulnificus?
V. vulnificus gastroenteritis occurs most commonly after consumption of contaminated raw oysters and can lead to septicemia with a 50-80% fatality rate.
We have shown that V. vulnificus, among many other species of the genera Vibrionacea, expresses sialic acid-like molecules. We found that Lineage I and Lineage II isolates, which are mostly clinical and environmental isolates respectively, encode lineage-specific alleles of the biosynthetic pathway for these sugars. Moreover, Lineage I isolates of mostly clinical origin have on average 40X higher density of expression of these molecules on their surface compared to Lineage II isolates of mostly environmental origin. A high density of sialic acid-like sugars on V. vulnificus may be significant during bloodstream infection by allowing the bacterium to evade innate immune responses.
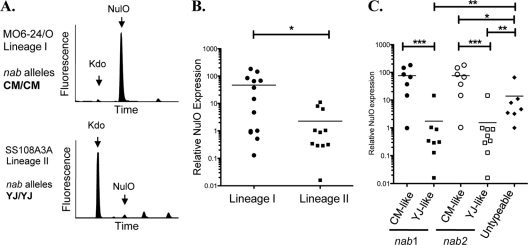
DMB HPLC analysis of diverse V. vulnificus isolates was carried out, and NulO expression levels were normalized to an internal control monosaccharide (Kdo) that is a part of the conserved core portion of LPS. (A) Raw HPLC data from strains representing the major V. vulnificus lineages (I and II) and common nab allele types (CMCP6 and YJ016). (B) Relative NulO expression levels in lineage I and II strains. (C) V. vulnificus isolates with CM-like alleles of nab genes have significantly higher levels of NulOs than isolates with YJ-like alleles. Most of the “untypeable” isolates do, in fact, express detectable NulOs but at levels that are intermediate compared to those of isolates with YJ-like or CM-like alleles. (B and C) (*, P < 0.05; * *, P < 0.01; * * *, P < 0.0001). Note the log scales.
Lewis Lab publications on the evolution of sialic acids and their biological functions
Ricaldi, J.N.; Matthias, M.A. Vinetz, J.M.; and Lewis, A.L. Expression of Sialic Acids and other Nonulosonic Acids in Leptospira. BMC Microbiol. 2012 Aug 1; 12(1):161. Epub ahead of print
Lewis, A.L.; Lubin, J.B.; Almagro-Moreno, S.; Argade, S.; Naidu, N.; Choudhury, B.; and Boyd, E.F. 2011. Genomic and Metabolic Profiling of Nonulosonic Acids in Vibrionaceae reveal Biochemical Phenotypes of Allelic Divergence in V. vulnificus. Appl Environ Microbiol. Aug 15;77(16):5782-93. http://www.ncbi.nlm.nih.gov/pubmed/21724895
Lewis, A.L.; Desa, N.; Hansen, E.E.; Knirel, Y.; Gordon, J.; Gagneux, P.; Nizet, V.; Varki, A. 2009. Innovations in Host and Microbial Sialic Acid Biosynthesis Revealed by Phylogenomic Prediction of Nonulosonic Acid Structure. Proc. Natl. Acad. Sci. U S A. 11;106(32):13552-7. Lewis_2009_PNAS.pdf
Lewis, A.L.; Varki, A. Evolutionary Considerations in Studying the Sialome: Sialic Acids and the Host-Pathogen Interface. Bioinformatics for Glycobiology and Glycomics: An Introduction. pgs 69-88. Wiley & Sons Ltd. 2009.
Lewis, A.L.; Hensler, M.; Nizet, V; Varki, A. 2006. The group B streptococcal sialic acid O-acetyltransferase is encoded by neuD, a conserved component of bacterial sialic acid biosynthetic gene clusters. J Biol Chem. 281(16):11186. Lewis_2006_JBC.pdf
Hayakawa, T.; Angata,T.; Lewis, A.L.; Mikkelsen, T.; Varki, N.; Varki, A. 2005. A human-specific gene in microglia. Science 309 (5741):1693. http://www.ncbi.nlm.nih.gov/pubmed/16151003